In many cases the liquid induces large and undesired adhesive forces. Inside a U-shaped wire frame with a movable hoop at the open end a thin liquid film eg.

Determination Of Solid Liquid Interfacial Energy Of Ni3sn2 Phase By Grain Boundary Groove Method In A Temperature Gradient Sciencedirect
Soap solution is spanned see figure 3.
Solid liquid interfacial energy. Solid-liquid interfacial energy is one of the key factors determining microstructure of casting metal and alloy since it affects growth velocity of the dendrite and its tip radius. Energy and interfacial energy changes as described for the liquid-to-solid transformation into the free energy change of a solid-solid transformation ΔGr r G V 3 3 4π 4πr2 γ strain energy Now lets derive the strain energy. If youre talking about a liquidsolid interface you can solve for the interfacial energy using the classical Youngs equation.
The surface energy of the solid and the interfacial tension between the solid and liquid are generally unknown. Solidliquid interfacial energy of the AgCu alloy as function of the average liquidus and solidus mole fractions of Cu. We simulate the melting with two methods the hysteresis one-phase approach and the solidliquid coexistence two-phase approach.
Solid-liquid interfacial energies are fundamental properties of materials and play a key role in material processing at the coexistence of solid and liquid phases such as for the processes of casting and crystal. Using the molecular dynamics simulation we employ spherical crystal nuclei embedded in the supercooled liquids to create an ideal model of a homogeneous nucleation. Over the last 40 years various attempts have been made to determine the value of the interfacial energy in a variety of materials.
The surface forces of the liquid film and the gravitational force of the movable hoop are in equilibrium. The surface energy of a liquid liquid-gaseous interface can be determined with a simple model experiment. Consider αβ solid-solid transformation as depicted below.
The solidliquid interface energy σ SL is defined as the reversible work required creating a unit area of the interface at constant temperature volume and chemical potentials and plays a critical role in many phase transformations. One of the most common techniques is to use grain boundary groove shapes. The solidliquid interfacial free energy of silicon was calculated by the method based on classical nucleation theory CNT where the molecular dynamic MD simulations were carried out and a series of cylindrical solid nuclei were equilibrated with undercooled liquid phase to create an ideal model of a homogeneous nucleation.
Although the anisotropy of the solid-liquid interfacial free energy for most alloy systems is very small it plays a crucial role in the growth rate morphology and crystallographic growth. The equilibrium contact angle has two extrema. IntroductionThe solid-liquid interfacial energy SL plays a critical role in phase transformations.
Interfaces comprised of a liquid interposed between two solids in close proximity are common in small-scale devices. But youll have to measure the liquidvapor interfacial energy using. Capillary fields in the form of interfacial energy distributions are exposed and measured on simulated microstructures in the form of equilibrated solidliquid grain boundary grooves GBGs.
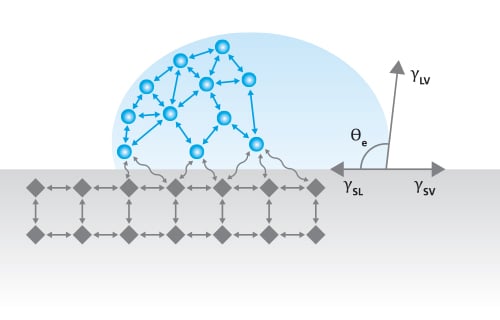
Simulated interfacial data also allow quantifiable comparison with analytic predictions of interfacial energy fields derived from sharp-interface thermodynamics. It is of interest therefore to model the way in which forces are developed in such an interface. Interfacial tension or a free interfacial energy can be attributed to each interface between solid S liquid L and its vapour V ie.
A force per unit length of triple line acts along each interface using the mechanical tension definition. To determine these values models that take into consideration different types of interaction between liquid and solid are used. A general model for predicting both coherent solidsolid and immiscible liquidliquid interfacial energies is derived from a thermodynamic point of view.
In this model the interfacial energy can be calculated only using the data about molar area. We carried out molecular dynamics MD simulations with the quantum-corrected SuttonChen Q-SC potential to compute the thermal equation of state EOS the solidliquid interfacial energy and melting properties of nickel. This new method is based on the classical nucleation theory.
Interface solid-liquid γSL solid-vapour γSV and liquid-vapour γLV. The horizontal dotted line only shows that at the eutectic point the solidliquid interfacial energies have the same values when the liquid is. We present a simple approach to calculate the solid-liquid interfacial free energy.
Surface Free Energy Measurements

Team Reveals Molecular Structure Of Water At Gold Electrodes Structured Water Molecular Molecular Structure
Rame Hart Instrument Co Monthly Newsletter

Estimation Of Solid Liquid Interfacial Tension Using Curved Surface Of A Soft Solid Pnas
